| - 2019 - | - 2018 - | - 2017 - | - 2016 - | - 2015 - | - 2014 - | - 2013 - | - 2012 - | - 2011 - | - 2010 - | - 2009 - |
2019
5. Modulating FOXO3 transcriptional activity by small, DBD-binding molecules.
|
|
FOXO transcription factors are critical regulators of cell homeostasis and steer cell death, differentiation and longevity in mammalian cells. By combined pharmacophore-modelling-based in silico and fluorescence polarization-based screening we identified small molecules that physically interact with the DNA-binding domain (DBD) of FOXO3 and modulate the FOXO3 transcriptional program in human cells. The mode of interaction between compounds and the FOXO3-DBD was assessed via NMR spectroscopy and docking studies. We demonstrate that compounds S9 and its oxalate salt S9OX interfere with FOXO3 target promoter binding, gene transcription and modulate the physiologic program activated by FOXO3 in cancer cells. These small molecules prove the druggability of the FOXO-DBD and provide a structural basis for modulating these important homeostasis regulators in normal and malignant cells. |
4. A formal [4 + 2] cycloaddition of sulfur-containing alkylidene heterocycles with allenic compounds.
Vojtěch Dočekal, Bedřich Formánek, Ivana Císařová and Jan Veselý Org. Chem. Front. 2019, 6, 3259.
|
|
In this study, we report an enantioselective synthesis of sulfur heterocycles containing dihydro-2H-pyran moiety. The synthetic strategy is based on quinidine catalyzed formal [4+2] cycloaddition between 3-alkylidene benzo[b]thiophenes and allenoates affording the corresponding cycloadducts in high yields (up to 95 %) with high enantioselectivity (up to 99 % ee). Moreover, cycloadducts can be isolated by simple filtration from the reaction media. |
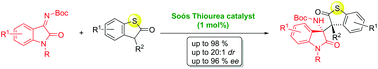
3. Highly enantioselective addition of sulfur-containing heterocycles to isatin-derived ketimines.
Michael Franc, Michal Urban, Ivana Císařová and Jan Veselý Org. Biomol. Chem. 2019, Advance Article.
|
|
In this study, we report a highly stereoselective addition of sulfur-containing heterocyclic compounds to isatin-derived ketimines efficiently catalyzed by cinchonidine-derived bifunctional tertiary aminothiourea (1 mol%). This organocatalytic methodology furnishes a new type of optically active heterocyclic compound with two adjacent chiral quaternary carbon stereocenters in good yield (up to 98%), with excellent diastereoselectivity (up to 20 : 1 dr) and high enantioselectivity (up to 95% ee). |
2. Formal [3+2] cycloaddition of vinylcyclopropane azlactones to enals using synergistic catalysis.
|
|
The present study reports the asymmetric cyclization of enals with vinylcyclopropane azlactones efficiently catalyzed by combination of achiral Pd(0) complexes and chiral secondary amines. Corresponding spirocyclic azlactones were produced in high yields with moderate diastereoselectivities and excellent enantioselectivities. This protocol provides an efficient and easy-performed route to spirocyclic scaffold, and densely functionalized cyclopentanes containing quaternary carbon center. |
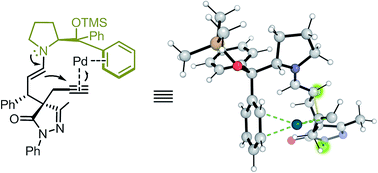
1. Proline Bulky Substituents Consecutively Act as Steric Hindrances and Directing Groups in a Michael/Conia-Ene Cascade Reaction under Synergistic Catalysis.
|
|
In this study, we report a highly stereoselective and versatile synthesis of spiro pyrazolones, promising motifs that are being employed as pharmacophores. The new synthetic strategy merges organocatalysis and metal catalysis to create a synergis-tic catalysis using proline derivatives and Pd catalysts. This protocol is suitable for late-stage functionalization, which is very im-portant in drug discovery. Additionally, a thorough computational study proved to be very useful to elucidate the function of the different catalysts along the reaction, showing a peculiar feature: the -CPh2OSiMe3 group of the proline catalyst switches its role during the reaction. In the initial Michael reaction, this group plays its commonly-assumed role of bulky blocking group, but the same group generates π-Pd interactions and acts as a directing group in the subsequent Pd-catalyzed Conia-Ene reaction. This finding might be very relevant especially for processes with many steps, such as cascade reactions, in which functional groups are assumed to play the same role during all reaction steps. |
2018
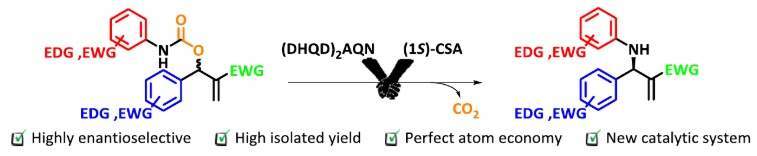
3. Organocatalytic Allylic Amination of Morita–Baylis–Hillman Carbonates.
|
|
An organocatalytic asymmetric allylic amination of Morita–Baylis–Hillman carbonates with aromatic amines in the presence of β-isocupreidine is described. Chiral allylic amines were obtained in almost quantitative yields (90–96%) with moderate enantioselectivity. Recrystallization afforded products in good yields (45–73%) and high optical purity (82–99% ee). This method provides a facile and efficient route to obtain optically active β-lactams, including the building block of the cholesterol-lowering drug Ezetimibe.
|
2.·Decarboxylative Organocatalytic Allylic Amination of Morita‐Baylis‐Hillman Carbamates.
Vojtěch Dočekal, Michal Šimek, Martin Dračínský and Jan Veselý·Chem. Eur. J.·2018, 5124,·3441-13445;
|
|
The present study reports the organocatalytic asymmetric allylic amination of Morita‐Baylis‐Hillman carbamates efficiently catalyzed with chiral amine in the presence of Brønsted acid. Chiral allylic amines were produced in high yields (up to 98%) and enantioselectivities (up to 97% ee). This method provides an efficient and easily‐performed route to prepare α‐methylene‐β‐lactams, and other optically active β‐lactams, such as the cholesterol‐lowering drug Ezetimibe.
|
1. Synergistic formal ring contraction for the enantioselective synthesis of spiropyrazolones.
|
|
The rapid generation of molecular complexity from simple reactants is a key challenge in organic synthesis. Spiro compounds, underrepresented 3D motifs in chemical libraries, represent a challenge due to the creation of spiro quaternary carbon and the need to control the 3D shape in one step. Herein, we report the first ring contraction/formal [6 + 2] cycloaddition using synergistic Pd(0)/secondary amine catalysis, obtaining [5,5]-spiropyrazolone derivatives in excellent yields and stereoselectivities. We demonstrate that this reaction has a broad scope of early and late stage derivatization that will benefit the creation of highly valuable chemical libraries using spiropyrazolone motifs. We detected the key palladium activated intermediate in its protonated form by mass spectrometry and characterized its structure by infrared spectroscopy and DFT calculations, allowing us to propose a conceivable mechanistic pathway for this reaction. |
2017
4. Asymmetric Allylic Amination of Morita–Baylis–Hillman Carbonates with Silylated tert‐Butylhydroxycarbamate Derivatives.
Martin Kamlar, Ivana Císařová, Simona Hybelbauerová, Jan Veselý Eur. J. Org. Chem., 2017,1926.
|
|
The enantioselective amination of Morita–Baylis–Hillman carbonates with silylated derivatives of tert-butylhydroxycarbamates using β-isocupreidine as an organocatalyst was investigated. The corresponding products were obtained in good yields with high enantioselectivities. The scope and limitations of the reported protocol were explored, and representative transformations of the adducts toward heterocyclic compounds were highlighted.
|
3. Molecular basis of the 14-3-3 protein-dependent activation of yeast neutral trehalase Nth1.
|
|
Molecular basis of the 14-3-3 protein-dependent activation of yeast neutral trehalase Nth1·The 14-3-3 proteins, a family of highly conserved scaffolding proteins ubiquitously expressed in all eukaryotic cells, interact with and regulate the function of several hundreds of partner proteins. Yeast neutral trehalases (Nth), enzymes responsible for the hydrolysis of trehalose to glucose, compared with trehalases from other organisms, possess distinct structure and regulation involving phosphorylation at multiple sites followed by binding to the 14-3-3 protein. Here we report the crystal structures of yeast Nth1 and its complex with Bmh1 (yeast 14-3-3 isoform), which, together with mutational and fluorescence studies, indicate that the binding of Nth1 by 14-3-3 triggers Nth1’s activity by enabling the proper 3D configuration of Nth1’s catalytic and calcium-binding domains relative to each other, thus stabilizing the flexible part of the active site required for catalysis. The presented structure of the Bmh1:Nth1 complex highlights the ability of 14-3-3 to modulate the structure of a multidomain binding partner and to function as an allosteric effector. Furthermore, comparison of the Bmh1:Nth1 complex structure with those of 14-3-3:serotonin N-acetyltransferase and 14-3-3:heat shock protein beta-6 complexes revealed similarities in the 3D structures of bound partner proteins, suggesting the highly conserved nature of 14-3-3 affects the structures of many client proteins. |
2. Highly Diastereo‐ and Enantioselective Synthesis of α‐Spiro‐δ‐lactams by an Organocascade Reaction.
|
|
An asymmetric synthesis of α-spiro-δ-lactams by an organocascade reaction of easily accessible starting materials has been accomplished. The catalytic sequence begins with enantioselective Michael addition of a β-ketoamide to an α,β-unsaturated aldehyde, catalyzed by a secondary amine catalyst, and is followed by hemiaminal annulation. Optically enantiopure compounds with three stereogenic centres are obtained in good yields and excellent selectivities (up to >20:1 dr and up to >99 % ee).
|
1. The enantioselective addition of 1-fluoro-1-nitro(phenylsulfonyl)methane to isatin-derived ketimines.
M. Urban, M. Franc, M. Hofmanová, I. Císařová and J. Veselý Org. Biomol. Chem., 2017,15, 9071.
|
|
An asymmetric synthesis of α-spiro-δ-lactams by an organocascade reaction of easily accessible starting materials has been accomplished. The catalytic sequence begins with enantioselective Michael addition of a β-ketoamide to an α,β-unsaturated aldehyde, catalyzed by a secondary amine catalyst, and is followed by hemiaminal annulation. Optically enantiopure compounds with three stereogenic centres are obtained in good yields and excellent selectivities (up to >20:1 dr and up to >99 % ee). |
2016
1. Stereoselective Synthesis of Ezetimibe via Cross-Metathesis of Homoallylalcohols and α-Methylidene-β-Lactams.
|
|
Ru-catalyzed cross-metathesis (CM) reaction between β-arylated α-methylidene-β-lactams and terminal olefins was developed. The CM reaction is effectively catalyzed with Hoveyda–Grubbs second-generation catalyst affording corresponding α-alkylidene-β-aryl-β-lactams in good isolated yields (41–83%) with exclusive Z-selectivity. The developed protocol was successfully applied for stereoselective preparation of Ezetimibe, the commercial cholesterol absorption inhibitor.
|
2015
3. Expanding the scope of Metal-Free enantioselective allylic substitutions: Anthrones.
|
|
The highly enantioselective asymmetric allylic alkylation of Morita–Baylis–Hillman carbonates with anthrones is presented. The reaction is simply catalyzed by cinchona alkaloid derivatives affording the final alkylated products in good yields and excellent enantioselectivities. |
2.·Bifunctional (Thio)urea–Phosphine Organocatalysts Derived from D-Glucose and α-Amino Acids and Their Application to the Enantioselective Morita–Baylis–Hillman Reaction.
I. Gergelitsová, J. Tauchman, I. Císařová, J. Veselý Synlett 2015, 26, 2690-2696.
|
|
Novel (thio)urea–tertiary phosphines were developed for use as bifunctional organocatalysts readily available from naturally occurring molecules: saccharides and amino acids. The efficiency of the organocatalysts was demonstrated in the asymmetric Morita–Baylis–Hillman (MBH) reaction of aromatic aldehydes with acrylates. The MBH products were obtained in good yields (up to 85%) and with high enantioselectivities (up to 87% ee).
|
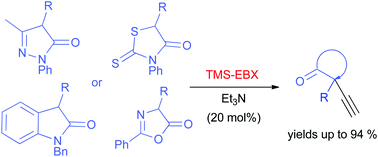
1. Alkynylation of heterocyclic compounds using hypervalent iodine reagent.
M. Kamlar, I. Císařová and J. Veselý Org. Biomol. Chem. 2015, 13, 2884-2889.
|
|
The alkynylation of various nitrogen- and/or sulphur-containing heterocyclic compounds using hypervalent iodine TMS-EBX by utilization of tertiary amines under mild conditions is described. The developed metal-free methodology furnishes the corresponding alkynylated heterocycles bearing quaternary carbon in high yields. |
2014
5. One-pot preparation of chiral carbacycles from Morita-Baylis-Hillman carbonates via asymmetric allylic alkylation/olefin metathesis sequence.
Putaj, P.; Tichá, I.; Císařová, I.; Veselý, J.·Eur. J. Org. Chem. 2014, 30, 6615-6620.
|
|
An operationally simple, one‐pot synthetic protocol for the formation of all‐carbon, highly substituted five‐ and six‐membered rings is described. In this two‐step procedure, an asymmetric allylic alkylation (AAA) of Morita–Baylis–Hillman (MBH) carbonates with allylmalononitrile, catalyzed by a chiral tertiary amine, is followed by a ring‐closing alkene metathesis (RCM) reaction. Products are obtained in high yields, and an excellent level of optical purity of some of the target compounds is achieved after just a single recrystallization.
|
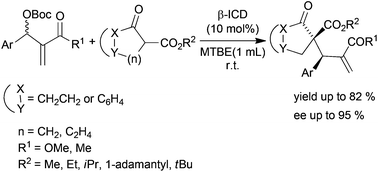
4. Organocatalytic enantioselective allylic alkylationof MBH carbonates with β-keto esters.
Kamlar, M.; Hybelbauerová, S.; Císařová, I.; Veselý, J. Org. Bimol. Chem. 2014, 12, 5071-5076.

The highly stereoselective allylic alkylation of Morita–Baylis–Hillman carbonates with β-ketoesters catalysed by β-ICD is described. The corresponding products containing two adjacent quaternary and tertiary carbon centers were obtained in good yields with high diastereoselectivity (up to 10 : 1 dr) and enantioselectivity (up to 95% ee).
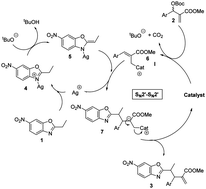
3. Synergistic catalysis: highly diastereoselective benzoxazole addition to Morita–Baylis–Hillman carbonates.

An expedited method has been developed for the diastereoselective synthesis of highly functionalized alkyl-azaarene systems with good yields and high diastereoselectivities (>15 : 1 dr). The methodology includes a synergistic catalysis event involving organometallic (10 mol% AgOAc) activation of an alkyl azaarene and Lewis base (10 mol% DABCO) activation of a Morita–Baylis–Hillman carbonate. The structure and relative configuration of a representative product were confirmed by X-ray analysis.
2. Organocatalytic Preparation of Substituted Cyclopentanes: A Mechanistic Study.
Tsybizova, A.; Remeš, M.; Veselý, J.; Roithová, J. J. Org. Chem. 2014, 79, 1563-1570.

The reaction mechanism of a tandem conjugate addition/α-alkylation of enals leading to functionalized cyclopentanes catalyzed by O-trimethylsilyldiphenylprolinol was investigated by mass spectrometry, NMR spectroscopy, and DFT calculations. We have shown that the high stereoselectivity of the reaction depends on the energy discrimination between the two stereoisomers formed by the condensation of the α,β-unsaturated aldehyde (cinnamaldehyde) and the catalyst. The stereoselectivity of this step depends on the solvent used.Â

1. Enantioselective methodologies using N-carbamoyl-imines.
Veselý, J.; Rios, R. Chem. Soc. Rev. 2014, 43, 611-630.

Nucleophilic addition to carbon–nitrogen double bonds (imines) represents one of the most common strategies for the synthesis of amine derivatives. In order to circumvent the problem associated with low reactivity of imines in nucleophilic addition, various imines with electron-withdrawing groups at nitrogen have been studied, and many of them were successfully applied in asymmetric methodologies. Especially N-carbamoyl imines were found to be useful in the enantioselective synthesis of various organic compounds, due to their increased reactivity toward nucleophiles as well as limited difficulties connected with the removal of the carbamoyl moiety in target molecules. The aim of this review is to cover enantioselective methods based on N-carbamoyl imines, focusing on synthetically useful protocols.
2013
5. The modulation of aldose reductase inhibition by halogen bond tuning.

In this paper, we studied a designed series of aldose reductase (AR) inhibitors. The series was derived from a known AR binder, which had previously been shown to form a halogen bondbetween its bromine atom and the oxygen atom of the Thr-113 side chain of AR. In the series, the strength of the halogen bond was modulated by two factors, namely bromine–iodine substitution and the fluorination of the aromatic ring in several positions. The role of the single halogen bond in AR–ligand binding was elucidated by advanced binding free energy calculations involving the semiempirical quantum chemical Hamiltonian. The results were complemented with ultrahigh-resolution X-ray crystallography and IC50 measurements. All of the AR inhibitors studied were shown by X-ray crystallography to bind in an identical manner. Further, it was demonstrated that it was possible to decrease the IC50 value by about 1 order of magnitude by tuning the strength of the halogen bond by a monoatomic substitution. The calculations revealed that the protein–ligand interaction energy increased upon the substitution of iodine for bromine or upon the addition of electron-withdrawing fluorine atoms to the ring. However, the effect on the binding affinity was found to be more complex due to the change of the solvation/desolvation properties within the ligand series. The study shows that it is possible to modulate the strength of a halogen bond in a protein–ligand complex as was designed based on the previous studies of low-molecular-weight complexes.

4. Enantioselective organocatalytic synthesis of novel sulfur-containing spirocyclic compounds.
Géant, P.-Y.; Urban, M.; Remeš, M., Císařová, I.; Veselý, J. Eur. J. Org. Chem. 2013, 7979-7988.

Two different enantioselective organocatalytic cascade reactions to form new sulfur‐containing spirocyclic scaffolds are described. In the first approach, benzothiophen‐2‐one and enals react in the presence of a secondary amine catalyst through a Michael/Michael/Aldol sequence to afford the final spiro‐cyclohexene carbaldehydes in good yields (up to 68 %) and with excellent selectivities [20:1 diastereomeric ratio (dr), up to 99 % ee]. In the second approach, the double Michael addition of benzothiophen‐2‐one to aromatic dienones with primary amine catalysis produces the corresponding spiro‐cyclohexanones in good yields (up to 76 %) and with moderate‐to‐high selectivities (up to 12:1 dr, up to 90 % ee). Moreover, the use of N‐phenylrhodanine as the bis‐nucleophile for these reactions also allowed the formation of the corresponding spirocyclic adducts.

3. Organocatalytic alkynylation of densely functionalized monofluorinated derivatives: C(sp3)–C(sp) coupling.
Kamlar, M.; Putaj, P.; Veselý, J. Tetrahedron Lett. 2013, 54, 2097-2100.

Organocatalytic alkynylation of nucleophilic fluorocarbons using hypervalent iodine compounds under cinchona-based catalysis, namely using O-allyl N-anthracenyl cinchona alkaloid derivative II, is described. The reaction gives the final fluoro-propargyl compounds in good to excellent yields (up to 91%) and with moderate to low enantioselectivity (up to 61% ee). The reaction represents the first example of the use of hypervalent iodine compounds for the construction of fluorinated compounds and opens access to the preparation of biologically attractive compounds such as 1,2,3-triazoles.

2. Highly enantioselective organocatalytic α‐selenylation of aldehydes using hypervalent iodine compounds.
Kamlar, M.; Veselý, J. Tetrahedron: Asymmetry 2013, 24, 254‐259.

The highly enantioselective organocatalytic α-selenylation reaction of aldehydes using a hypervalent iodine compound as an oxidative agent from commercially available phenyl diselenide under mild oxidative conditions is described. This transformation affords α-selenyl aldehydes in good yields and with excellent enantioselectivities. By using hypervalent iodine compounds, it opens up a suitable and alternative way for the preparation of biologically active building blocks such as β-hydroxy alcohols, α-amino acids, and α-hydroxy esters.

1. Enantioselective Organocatalytic Amination of Pyrazolones.
Šimek, M.; Remeš, M.; Veselý, J.; Rios, R. Asian J. Org. Chem. 2013, 2, 64‐68.

The organocatalytic enantioselective amination of pyrazolones is reported. The reaction between pyrazolones and diazodicarboxylates is simple and is catalyzed by quinine to afford the final aminopyarazolone derivatives in excellent yields and good enantioselectivity.

2012
3. Structure‐aided design of novel inhibitors of HIV protease based on a benzodiazepine scaffold.

HIV protease is a primary target for the design of virostatics. Screening of libraries of non-peptide low molecular weight compounds led to the identification of several new compounds that inhibit HIV PR in the low micromolar range. X-ray structure of the complex of one of them, a dibenzo[b,e][1,4]diazepinone derivative, showed that two molecules of the inhibitor bind to the PR active site. Covalent linkage of two molecules of such a compound by a two-carbon linker led to a decrease of the inhibition constant of the resulting compound by 3 orders of magnitude. Molecular modeling shows that these dimeric inhibitors form two crucial hydrogen bonds to the catalytic aspartates that are responsible for their improved activity compared to the monomeric parental building blocks. Dibenzo[b,e][1,4]diazepinone analogues might represent a potential new class of HIV PIs.

2. Highly enantioselective organocatalytic formation of functionalized cyclopentane derivatives via tandem conjugate addition/α‐alkylation of enals.
Remeš, M.; Veselý, J. Eur. J. Org. Chem. 2012, 20, 3747‐3752.

An enantioselective tandem reaction between dialkyl 2‐haloethylmalonates and aromatic enals catalyzed by secondary amine catalysts is described. The reaction proceeds through a Michael/α‐alkylation reaction sequence to afford the final 1,1,2,3‐tetrasubstituted cyclopentanes in good yields (up to 75 %) with high diastereoselectivity and enantioselectivity (up to 20:1 dr and 93 % ee). Moreover, the Michael/α‐alkylation reaction also proceeds with a low catalyst loading (5 mol‐%) with only a slight influence on selectivity and O‐ and N‐alkylation products were not observed.

1. Organocatalytic enantioselective α‐alkylation of aldehydes.
Veselý, J.; Rios, R. Chem. Cat. Chem. 2012, 4, 942‐953.

Organocatalytic intermolecular α‐alkylation has been a long awaited prize for organocatalytic chemists. Recently several research groups have reported several methodologies with high levels of stereoselectivity. In this Minireview we aim to describe the efforts made in this field in recent years.
2011
3. Highly enantioselective organocatalytic cascade reaction for the synthesis of piperidines and oxazolidines.

The synthesis of piperidines and piperidines derivatives in enantiopure fashion has been a challenging goal for organic chemists. In this report we developed a nice cascade reaction for piperidine derivatives based in an amidomalonate Michael addition to enals followed by an intramolecular hemiaminal formation with good yields and enantioselectivities. Moreover we studied the ‘in situ’ intramolecular cyclization of this hemiaminals with alcohols forming fused piperidine–oxazolidines.

2. Asymmetric Aza‐Morita‐Baylis‐Hillman‐Type Reactions: The Highly Enantioselective Reaction between Unmodified α,β‐Unsaturated Aldehydes and N‐Acylimines by Organo‐co‐catalysis.
Číhalová, S.; Dziedzic, P.; Córdova, A.; Veselý, J. Adv. Synth. Catal. 2011, 353 (7), 1096‐1108.
 |
The highly enantioselective organo‐co‐catalytic aza‐Morita–Baylis–Hillman (MBH)‐type reaction between N‐carbamate‐protected imines and α,β‐unsaturated aldehydes has been developed. The organic co‐catalytic system of proline and 1,4‐diazabicyclo[2.2.2]octane (DABCO) enables the asymmetric synthesis of the corresponding N‐Boc‐ and N‐Cbz‐protected β‐amino‐α‐alkylidene‐aldehydes in good to high yields and up to 99% ee. In the case of aza‐MBH‐type addition of enals to phenylprop‐2‐ene‐1‐imines, the co‐catalytic reaction exhibits excellent 1,2‐selectivity. The organo‐co‐catalytic aza‐MBH‐type reaction can also be performed by the direct highly enantioselective addition of α,β‐unsaturated aldehydes to bench‐stable N‐carbamate‐protected α‐amidosulfones to give the corresponding β‐amino‐α‐alkylidene‐aldehydes with up to 99% ee. The organo‐co‐catalytic aza‐MBH‐type reaction is also an expeditious entry to nearly enantiomerically pure β‐amino‐α‐alkylidene‐amino acids and β‐amino‐α‐alkylidene‐lactams (99% ee). The mechanism and stereochemistry of the chiral amine and DABCO co‐catalyzed aza‐MBH‐type reaction are also discussed.
|
1. Enantioselective Organocatalytic Synthesis of 5 and 6 Membered Heterocycles.
Veselý, J.; Rios, R., Curr. Org. Chem, 2011,15, 4046‐4082.

During last decade the field of Organocatalysis emerged as one of the cornerstones of organic asymmetric synthesis. The use of organocatalysis has some advantages such as metal free reactions, environmentally friendly conditions, does not need to use dry solvents or inert atmosphere, avoid the use of protecting groups, etc… For these reasons, several research groups have focused their efforts on the development of new organocatalytic methodologies that render the final products in excellent yields and stereoselectivities. Concretely, the organocatalytic synthesis of 5 and 6 membered heterocycles has attracted much attention. In this review we have the aim to describe the different organocatalytic methodologies developed since in the synthesis of 5 and 6 heterocycles.
2010
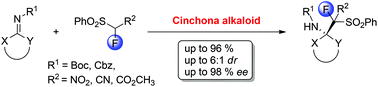
1. Highly enantioselective addition of 1‐fluoro‐1‐nitro(phenylsulfonyl)methane to α,β‐unsaturated aldehydes.

An organocatalytic, highly enantioselective addition of 1‐fluoro‐1‐nitro(phenylsulfonyl)methane to α,β‐unsaturated aldehydes is reported. The reaction is simply catalyzed by secondary amines and furnishes the corresponding fluorinated derivatives in good yields, with moderate diastereoselectivities and excellent enantioselectivities. The absolute configuration of the major diastereomers was unambiguously ascertained by X‐ray diffraction analysis.

2009
3. Highly enantioselective fluoromalonate addition to α,β‐unsaturated aldehydes.

A highly enantioselective organocatalytic fluoromalonate addition to α,β-unsaturated aldehydes is reported. The reaction is catalyzed by simple and commercially available secondary amines, affording the corresponding 1,4-adducts with high yields and enantioselectivities.

2. Highly enantioselective Aza‐Baylis‐Hillman‐Type Reaction of α,β‐Unsaturated Aldehydes with In Situ Generated N‐Boc‐ and N‐Cbz‐Imines.
Číhalová, S.; Remeš, M.; Císařová, I.; Veselý, J. Eur. J. Org. Chem. 2009, 6277‐6280.

Chiral β‐amino carbonyl compounds bearing an α‐alkylidene group were easily synthesized under mild and simple conditions from α,β‐unsaturated aldehydes and α‐amido sulfones in good yields and diastereoselectivities and excellent enantioselectivities

1. Highly enantioselective organocatalytic synthesis of piperidines. Formal synthesis of (‐)‐Paroxetine.

A highly enantioselective organocatalytic synthesis of piperidines is reported. The reaction is catalyzed by simple and commercially available secondary amines, affording the corresponding adducts with high yields and enantioselectivities. Moreover, this reaction is used for the formal synthesis of (−)-Paroxetine, a blockbuster drug, in only three steps.






![Graphical abstract: A formal [4 + 2] cycloaddition of sulfur-containing alkylidene heterocycles with allenic compounds](https://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C9QO00886A&imageInfo.ImageIdentifier.Year=2019)


![Graphical abstract: Formal [3+2] cycloaddition of vinylcyclopropane azlactones to enals using synergistic catalysis](https://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8CC06500D)